This post is a part of the Chemistry Carnival hosted by Chemical & Engineering News in celebration of the International Year of Chemistry. Check there later in the week to see what others have blogged or look for the #chemcarnival hashtag on Twitter.
This post is a part of the Chemistry Carnival hosted by Chemical & Engineering News in celebration of the International Year of Chemistry. Check there later in the week to see what others have blogged or look for the #chemcarnival hashtag on Twitter.
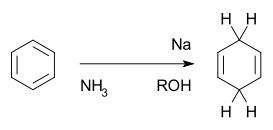
I spent nearly a decade of my life doing organic chemistry. Sometimes I defied the norm and carried out reactions in water. But I never quite got over my first. My first real reaction in a research laboratory was a Birch reduction.
This is a simplified scheme (and I lost my last working copy of ChemDraw when my computer before last crashed). I was doing the reaction on a steroid, converting the aromatic A ring of an estrogen to an androgen. But if you’re interested in the reaction details, other websites can tell you all you ever wanted to know about aromaticity, ammonia, lithium, and the quench with ethanol.
What sticks in my mind was the process of setting up and carrying out that reaction. I careful prepared dry glassware, I assembly of the glassware from the bottom up. I made sure I had enough dry ice and liquid nitrogen, and made sure that the flow of nitrogen gas was both slow and steady. Most of the techniques I had to learn to run every other reaction in my chemistry career started with that Birch reduction.
I tried to be patient as ammonia gas slowly condensed in my reaction mixture. I added bits of soft lithium metal that had yielded to snips of the scissors. I watched the solution deepen to brilliant blue as unpaired electrons worked their magic. Little did I know that the rest of my chemistry career would be filled with white powders or vaguely yellow goo.
Fortunately, my adviser watched me like a hawk, making sure I didn’t quench the reaction with ethanol too soon or too quickly. I’d learn that lesson the hard way in my next laboratory, when I got impatient from waiting hours for a reaction mixture to warm to room temperature. I assume that most chemists learn the hard way at least once?
Occasionally my former chemistry colleagues and friends will ask me, “Do you miss the lab? Do you miss doing reactions?” Day-to-day, I don’t. I’m pretty happy in front of my computer. But occasionally I am nostalgic for the rhythm and physical ritual of setting up a Birch reduction. At the end of the day, I always felt satisfied that I’d both worked hard and produced a white, fluffy powder.
Image Credits: birch forest photo by tumpikuja via iStockphoto; synthetic scheme by V8rik via Wikimedia Commons