Global health has been on my mind again recently. An article I wrote for Nature Reviews Drug Discovery (subscription required) examines efforts to find new drugs for tuberculosis.
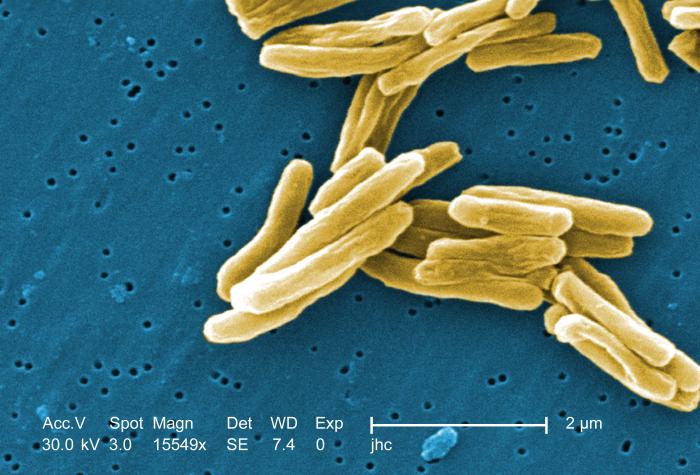
TB is a wily organism that finds a way to wall itself off in the body. Under the best circumstances, knocking out “the best” TB, the drug susceptible variety, requires 6 to 9 months of antibiotics. That’s a long time to stay on drugs, particularly in a developing country where the health clinic could be miles away. But if people start treatment without completing it, drug resistant disease can develop. With multi-drug resistant (MDR) or extensively drug resistant (XDR) disease, that prognosis is far more uncertain. The treatments being given in these cases don’t have much scientific validation to show that they work. In some cases of XDR TB, doctors are cutting out portions of diseased lung tissue from patients because there’s not much else that they can do.
My article mostly talks about the drug discovery challenges: how do you find a new drug for an old disease particularly when half a century ago we thought this problem was solved. But there’s another piece of this story that didn’t make it into the article: how do you then do the clinical studies to test these drugs among the people who need these drugs most?
In countries where health care systems are already taxed and where doctors and nurses may not be trained to carry out clinical trial protocols, there’s an additional wrinkle. TB treatments– somewhat uniquely– are written into health care policy in countries around the world. Almost all other diseases have recommended treatments, but the final decision belongs with the medical professionals who see the patients. What does that mean for TB clinical trials? Many more layers of red tape to set up a clinical trial. Not only do researchers have to work out trial protocols with the hospitals, they also have to get approvals from local and national authorities to modify the existing TB treatment protocols. I was amazed at the amount of coordination that’s involved.
Learn more about the search for new TB treatments at the Global Alliance for TB Drug Development.

Interesting, Sarah! As your example illustrates, thorough reporting always leaves those “wait, there’s more to the story” cliffhangers that don’t fit into the original story. Perhaps you’ll write another about story about clinical trials in 3rd world countries?
What a good idea! Hmm, must think about that one.